
In both cases only the OGs and GCs with a transcript abundance-based prevalence higher than 10% were considered in order to avoid spurious associations. Associations were detected as statistically significant differences in transcript abundance by Wilcoxon tests for depth layers and polar/non-polar regions (p 0.6 and p < 0.05, after Holm correction for multiple comparisons).
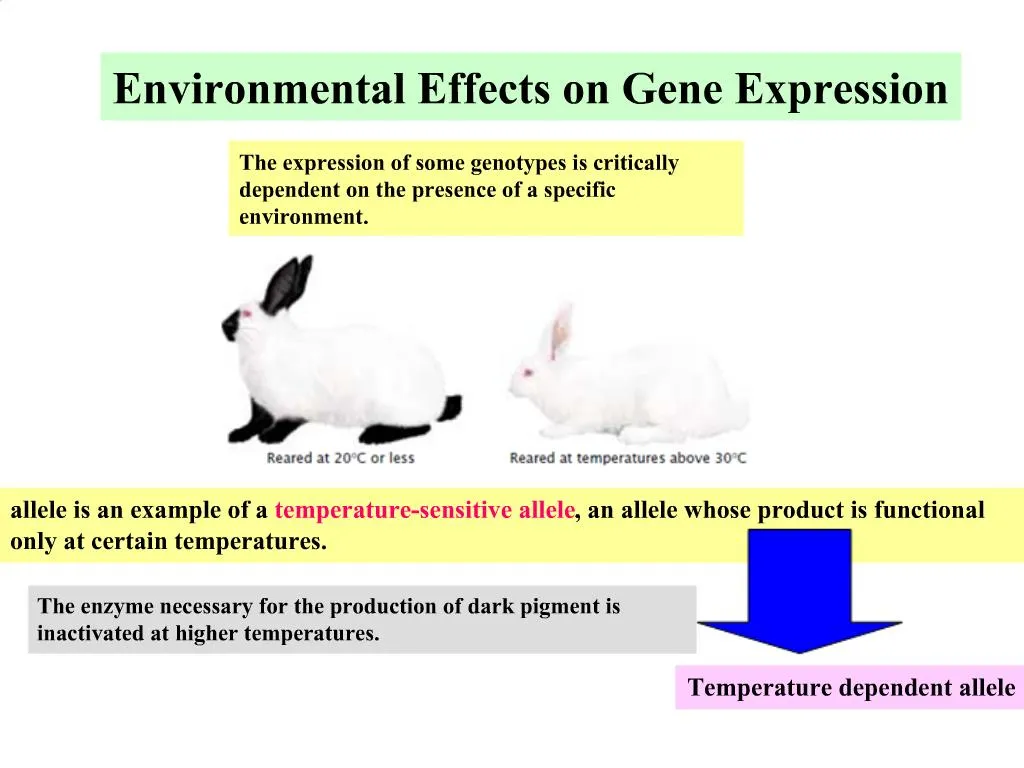
The numbers of OGs and GCs with significant associations of transcript abundances to depth layers (C) and polar/non-polar regions and (D) to environmental variables are shown. Prevalence distributions are shown in the side and upper panels. Gene abundance-based prevalence versus transcript abundance-based prevalence (i.e., number of samples in which detected) for (A) eggNOG-based orthologous groups (OGs) and (B) de novo gene clusters (GCs) based on the 122 paired metagenomes and metatranscriptomes. Furthermore, we find the relative contribution of gene expression changes to be significantly lower in polar than in non-polar waters and hypothesize that in polar regions, alterations in community activity in response to ocean warming will be driven more strongly by changes in organismal composition than by gene regulatory mechanisms. We examine gene expression changes and community turnover as the underlying mechanisms shaping community transcriptomes along these axes of environmental variation and show how their individual contributions differ for multiple biogeochemically relevant processes.
Here, we present a dataset of 187 metatranscriptomes and 370 metagenomes from 126 globally distributed sampling stations and establish a resource of 47 million genes to study community-level transcriptomes across depth layers from pole-to-pole. Despite recent advances in understanding their taxonomic and genomic compositions, little is known about how their transcriptomes vary globally. The authors have declared no competing interest.Ocean microbial communities strongly influence the biogeochemistry, food webs, and climate of our planet. We present confirmatory evidence for the presence of TMPRSS2, CD147, and GRP78 protein in vitro in airway epithelial cells and confirm broad in situ protein expression of CD147 in the respiratory mucosa.Ĭollectively, our data suggest the presence of a mechanism dynamically regulating ACE2 expression in human lung, perhaps in periods of SARS-CoV-2 infection, and also suggest that alternate receptors for SARS-CoV-2 exist to facilitate initial host cell infection. Consistent with gene expression, rare ACE2 protein expression was observed in the airway epithelium and alveoli of human lung. We demonstrate absent to low ACE2 promoter activity in a variety of lung epithelial cell samples and low ACE2 gene expression in both microarray and scRNAseq datasets of epithelial cell populations. To determine the expression and in situ localization of candidate SARS-CoV-2 receptors in the respiratory mucosa, we analyzed gene expression datasets from airway epithelial cells of 515 healthy subjects, gene promoter activity analysis using the FANTOM5 dataset containing 120 distinct sample types, single cell RNA sequencing (scRNAseq) of 10 healthy subjects, immunoblots on multiple airway epithelial cell types, and immunohistochemistry on 98 human lung samples. Additional host molecules including ADAM17, cathepsin L, CD147, and GRP78 may also function as receptors for SARS-CoV-2. ACE2 and TMPRSS2 have recently been implicated in SARS-CoV-2 viral infection.

SARS-CoV, the agent responsible for the 2003 SARS outbreak, utilizes ACE2 and TMPRSS2 host molecules for viral entry.

In December 2019, SARS-CoV-2 emerged causing the COVID-19 pandemic.


 0 kommentar(er)
0 kommentar(er)
